Overview
Chimeric Antigen Receptor T-Cell Therapy (CAR-T) is a cutting-edge form of cellular immunotherapy. This innovative treatment re-engineers a patient’s own T lymphocytes through genetic modification, enabling them to precisely recognize and target tumor cells for elimination.
The advent of CAR-T therapy has brought new hope for patients with hematologic malignancies and is showing promising potential in treating autoimmune diseases and solid tumors—opening new therapeutic avenues for individuals who previously lacked effective treatment options.
Applications of CAR-T Therapy
CAR-T therapy has demonstrated remarkable efficacy in multiple hematologic malignancies, including acute lymphoblastic leukemia (ALL), lymphoma, and multiple myeloma. Meanwhile, its applications are being actively explored in autoimmune diseases such as systemic lupus erythematosus and in solid tumors such as gastrointestinal cancers, offering new possibilities for broader patient populations.
GoBroad Healthcare Group has established a comprehensive CAR-T clinical research and treatment system, covering every stage from patient screening, cell manufacturing, and treatment administration to long-term follow-up. We not only provide routine CAR-T treatments for various hematologic malignancies but also actively advance pioneering clinical studies in autoimmune diseases and solid tumors—committed to extending the benefits of cellular immunotherapy to a wider range of patients.
Why Choose GoBroad?
GoBroad Healthcare Group was among the first in China to systematically develop and implement CAR-T cell therapy, continuously promoting the standardization and clinical innovation of this technology. Having performed several thousand CAR-T treatments, our team has accumulated extensive experience and achieved clinically validated efficacy and safety across diverse disease types.
Comprehensive Target Portfolio
GoBroad has built a mature CAR-T technology platform with an extensive range of therapeutic targets, enabling precision treatment from hematologic malignancies to solid tumors.
Targets for Hematologic Tumors:
- Single-target CAR-T: CD19, CD20, CD22, CD7, CD5, BCMA, GPRC5D, CLL1
- Dual-target CAR-T: Sequential CD19-22 CAR-T, Sequential CD19-20 CAR-T
Targets for Solid Tumors:
- Claudin18.2 CAR-T and others
Innovative Combination Strategies
GoBroad actively integrates CAR-T therapy with various conventional and emerging treatments—including chemotherapy, targeted therapy, and hematopoietic stem cell transplantation—to improve overall remission rates and long-term survival in relapsed or refractory patients.
Comprehensive Treatment and Care Management
Our integrated care model encompasses assessment, implementation, monitoring, and follow-up throughout the entire CAR-T treatment journey. From patient enrollment and cell preparation to therapy execution and rehabilitation, GoBroad provides personalized, systematized care supported by multidisciplinary collaboration to ensure safety, efficacy, and quality of long-term follow-up.
Multidisciplinary Collaboration (MDT)
GoBroad practices a patient-centered, multidisciplinary team approach, uniting hematology, integrated diagnostics, infectious disease imaging, respiratory medicine, and other specialties. This cross-disciplinary collaboration enables precise, systematic, and comprehensive diagnosis and treatment, forming the core strength of our cli
Our Achievements
For Relapsed/Refractory Leukemia
- CD19 CAR-T therapy for R/R B-ALL: Complete remission (CR) rate over 90%
- Sequential CD19 and CD22 CAR-T post-transplant relapse (B-ALL): 12-month and 18-month overall survival (OS) rates of 88.5% and 67.5%, respectively
- CD22 CAR-T after CD19 CAR-T failure: CR rate 71%, MRD negativity near 100%, outperforming international benchmarks
- Sequential CD19 and CD22 CAR-T in pediatric R/R B-ALL (Phase II): 18-month event-free survival (EFS) 79%, disease-free survival (DFS) 80%, OS 96%
- CD19 CAR-T for relapsed/refractory non-B-cell acute leukemia (AML, T-ALL): CR rate 62.5%
- Donor-derived CD7 CAR-T for R/R T-ALL: CR rate 90%, with 27-month median follow-up showing ORR 95% and CR 85%
- Donor-derived CD5 CAR-T for R/R T-ALL: 100% achieved CR or CRi by day 30 post-infusion
- Autologous unselected CD7 CAR-T for R/R T-ALL: 96% response rate, 85% achieving CR or CRi by day 30
For Relapsed/Refractory Lymphoma and Multiple Myeloma
- First global report: Triple-target CD19/CD20/CD22 CAR-T therapy in pediatric R/R Burkitt lymphoma achieved a 100% response rate and 86.9% CR at 18 months
- Autologous HSCT combined with CAR-T for R/R CNS B-cell lymphoma: ORR 74%, 1-year ORR 85.1%, 1-year PFS 44.5%, with controllable ICANS
- Donor CAR-T as preconditioning for allo-HSCT in R/R B-cell lymphoma: Median follow-up 249 days; CR 75%, 6-month OS and PFS 75% and 62.5%, respectively
The efficacy data are derived from published studies and GoBroad Healthcare Group’s long-term follow-up results. For medical reference only. Individual outcomes may vary depending on disease type and treatment plan. Please consult with a qualified physician for a personalized treatment strategy.
Related Reading
At GoBroad Shanghai Liquan Hospital, Prof. Su Li shared his valuable clinical experience, research achievements, and forward-looking perspectives in the treatment of multiple myeloma .
Q1. While BCMA-targeted CAR-T therapy has achieved breakthroughs in multiple myeloma, plasmablastic myeloma (PBM), as an aggressive subtype, has long lacked effective treatment options. What is the current status of diagnosis and treatment for PBM patients?
Prof. Su Li: Plasmablastic myeloma is a special subtype of myeloma with unique clinical characteristics. In clinical practice, a small proportion of patients can be diagnosed at disease onset, but the majority are only identified at the relapsed/refractory stage. The overall incidence remains relatively low, though it varies among centers. Our research has shown an incidence of approximately 3%–18%.
In terms of treatment, current outcomes for PBM remain unsatisfactory. Neither traditional chemotherapy nor novel therapeutic strategies have significantly improved remission or long-term survival. Existing clinical data suggest a median overall survival of about 10 months (range 6–12 months) for most patients, underscoring the urgent need for more effective approaches to improve prognosis and break through current clinical limitations.
Q2. How do the outcomes of BCMA CAR-T monotherapy compare with BCMA CAR-T combined with transplantation in PBM patients? What factors are usually considered when formulating a treatment strategy?
Prof. Su Li: The application and progress of CAR-T therapy in PBM must be understood within its biological context and clinical practice. Our earlier studies found that BCMA expression rises significantly in refractory/relapsed myeloma, especially with extramedullary involvement, providing a clear biological basis for BCMA-targeted CAR-T therapy.
Studies comparing single-target versus multi-target CAR-T strategies have shown that single-target approaches often result in early extramedullary relapse. In contrast, dual-target regimens, such as BCMA/CD19 or BCMA/GPRC5d, achieve higher MRD-negativity rates, deeper responses, and more durable remissions compared to BCMA monotherapy.
In clinical practice, CAR-T selection requires a comprehensive assessment of several factors. Pathological testing (immunohistochemistry, bone marrow flow cytometry, etc.) must first confirm antigen expression. For patients with BCMA-only expression, a BCMA/CD19 dual-target CAR-T is preferable. For those co-expressing BCMA and GPRC5d, a BCMA/GPRC5d dual-target approach is recommended. Dual-target regimens are especially advantageous in extramedullary disease, whereas single-target therapy may still be effective in purely intramedullary disease.
Another critical factor is prior CAR-T exposure. With growing adoption, some patients relapse after initial CAR-T therapy, often due to antigen loss or residual CAR-T immunogenicity, leading to diminished efficacy. For relapsed patients, switching to a CAR-T with a different construct is essential to avoid immune interference. Clinical practice has confirmed that such heterologous construct selection yields significant efficacy in short-term relapsed or expansion-failure patients, demonstrating its value in real-world treatment.
Q3. What challenges remain in applying CAR-T therapy more broadly in multiple myeloma, and how might they be overcome to benefit more patients?
Prof. Su Li: As a rare disease, PBM has a low incidence, and large-scale international studies are scarce. CAR-T therapy still faces several challenges:
- Accessibility: High costs and frequent out-of-pocket payment limit widespread adoption. Future innovations in technology or payment models could lower costs, enabling CAR-T to be used earlier rather than only at terminal stages. Our research shows that most patients receive CAR-T late in the disease course, where efficacy is more limited.
- Timing: Treatment line strongly affects outcomes. Later-line patients have weaker immune function, and their harvested T cells are often senescent or functionally impaired, reducing CAR-T manufacturing quality and in vivo expansion. Early T-cell collection, when cells are healthier, could improve outcomes.
- Construct design: Efficacy varies among patients even with the same CAR-T product. Beyond T-cell quality, antigen-binding affinity between CAR-T and tumor cells plays a role. Continued optimization of CAR design to enhance antigen recognition and binding is a key research focus.
- Combination strategies: Sequential or combinatorial approaches are under active investigation, such as CAR-T followed by autologous stem cell transplantation, or maintenance with immunomodulators or PD-1 inhibitors. These strategies may prolong remission and improve survival, and related studies are ongoing.


On June 1, 2025, the world's first randomized controlled trial (RCT) of CAR-T therapy for gastric cancer—"Claudin-18 isoform 2-specific CAR T-cell therapy (satri-cel) versus treatment of physician’s choice for previously treated advanced gastric or gastro-oesophageal junction cancer (CT041-ST-01): a randomised, open-label, phase 2 trial"—designed and led by Prof. Lin Shen’s team, was officially published in the prestigious international journal The Lancet (Impact Factor = 168.9). The study’s first author, Prof. Changsong Qi, also presented the positive findings in an oral session at the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, drawing widespread attention.

On June 3, the study results also made the headline on Nature’s official website.
Background
The CT041-ST-01 trial (NCT04581473) is the first randomized controlled clinical trial globally of CAR-T therapy targeting solid tumors. It evaluated the efficacy and safety of Satricabtagene Autoleucel (satri-cel)—a CLDN18.2-specific autologous CAR-T cell therapy—versus treatment of physician’s choice (TPC) in patients with previously treated advanced gastric or gastroesophageal junction cancer (G/GEJC).
CLDN18.2 is overexpressed in several gastrointestinal cancers and has emerged as a promising therapeutic target, especially in G/GEJC. Satri-cel showed encouraging results in a prior phase 1 trial involving patients with advanced G/GEJC who had failed standard therapies. The breakthrough results were published by Prof. Shen's team in Nature Medicine in June 2024 (Qi, C. et al. Nat. Med. 2024. doi: 10.1038/s41591-024-03037-z), laying a strong scientific foundation for advancing CAR-T in solid tumors. Building on these findings, the team launched the confirmatory phase 2 trial in CLDN18.2-positive G/GEJC patients who had failed at least two prior lines of therapy.
Study Design
This phase 2, open-label, multicenter, randomized controlled trial was conducted in China to compare the efficacy and safety of satri-cel versus standard therapies in CLDN18.2-positive (≥40% tumor cells with membrane staining intensity ≥2+) advanced G/GEJC patients who had failed at least two prior lines of therapy.
Eligible patients were randomized in a 2:1 ratio to receive:
- Satricel group: up to 3 infusions of satri-cel (2.5×10^8 cells per infusion),
- TPC group: treatment of physician’s choice (paclitaxel, docetaxel, irinotecan, nivolumab, or apatinib).
TPC patients were allowed to cross over to receive CT041 upon progression or intolerance, at the investigator’s discretion.
The primary endpoint was progression-free survival (PFS) assessed by Independent Review Committee (IRC). The key secondary endpoint was overall survival (OS).
Results
As of October 18, 2024, a total of 156 patients were randomized into the study, comprising the intent-to-treat (ITT) population, including 104 patients in the satri-cel group and 52 patients in the treatment of physician’s choice (TPC) group. A total of 88 patients (84.6%) in the satri-cel group and 48 patients (92.3%) in the TPC group received the study treatment, forming the modified intent-to-treat (mITT) population. Among them, 20 patients in the TPC group subsequently received CT041 infusion. All participants had previously received at least two lines of therapy, with 26.9% in the satri-cel group and 19.2% in the TPC group having received three or more lines. The proportion of peritoneal metastasis was 69.2% vs 59.6%, respectively.
In the ITT population:
Based on evaluation by the Independent Review Committee (IRC), satri-cel significantly prolonged progression-free survival (PFS) compared to the control group (median PFS: 3.25 months vs 1.77 months; HR: 0.37, 95% CI: 0.24–0.56; p < 0.0001), achieving the primary endpoint of the study. At the same time, overall survival (OS) showed a clear trend toward benefit (median OS: 7.92 months vs 5.49 months; HR: 0.69, 95% CI: 0.46–1.05; one-sided p = 0.0416). Notably, even with 15.4% (16 patients) in the satri-cel group not receiving cell infusion and approximately 40% (20 patients) in the TPC group later receiving satri-cel, the CT041 group still demonstrated over 30% reduction in mortality risk.
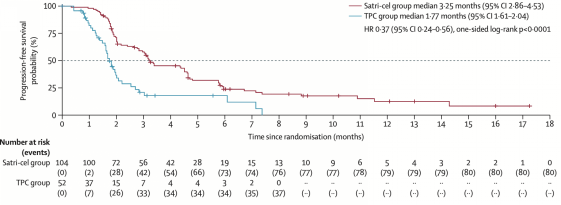
Primary Endpoint Achieved: PFS in ITT Population Shows Positive Results
In the mITT population (patients who actually received treatment): Based on IRC evaluation, median PFS in the satri-cel and TPC groups was 4.37 months vs 1.84 months (HR: 0.30, 95% CI: 0.19–0.47); median OS was 8.61 months vs 5.49 months (HR: 0.60, 95% CI: 0.38–0.94).
Therefore, among patients who actually received cell infusion, the therapeutic benefit of CT041 was even more pronounced.
It is worth noting that the median OS among the 20 TPC patients who subsequently received satri-cel was 9.20 months. Among all patients who received satri-cel infusion (n=108) from both groups, the median OS reached 9.17 months.
In terms of safety, satri-cel was generally well tolerated. Only 4 patients experienced grade 3 cytokine release syndrome (CRS); no grade 4 or 5 CRS occurred. No immune effector cell-associated neurotoxicity syndrome (ICANS) events were reported.
Conclusion
This study represents the first confirmatory randomized controlled trial of CAR-T therapy for solid tumors globally and marks a milestone in the field.
In CLDN18.2-positive G/GEJC patients who had failed at least two prior treatments, satri-cel significantly improved PFS and showed clinically meaningful OS benefits, with a manageable safety profile. The results strongly support satri-cel as a new third-line standard of care for advanced CLDN18.2-positive G/GEJC.
At the ASCO 2025 oral presentation, Prof. Changsong Qi told media:
"These results suggest that CLDN18.2 CAR-T therapy could transform the treatment landscape for advanced gastric cancer and offer a nearly unprecedented cell therapy option for solid tumors."


CAR-T cell therapy has achieved groundbreaking success in the treatment of hematologic malignancies, showcasing the immense potential of immunotherapy. As our understanding of immune mechanisms deepens, researchers have increasingly turned their attention to autoimmune diseases. Since B cells play a key role in the pathogenesis of many autoimmune disorders, CAR-T therapy—capable of precisely and deeply eliminating pathogenic B cells—has emerged as a novel and promising strategy. In this special feature, we invite Dr. Jinying Jin and Dr. Yajing Zhang to explore the latest progress and clinical applications of CAR-T therapy in the field of autoimmune diseases.
Current Landscape of Autoimmune Disease Treatment
Under normal circumstances, the immune system defends the body without attacking its own tissues. However, in autoimmune diseases, this recognition breaks down. The immune system mistakenly targets and attacks the body’s own cells—affecting joints, skin, glands, blood vessels, and multiple organ systems—leading to chronic inflammation and tissue damage. Common autoimmune diseases include systemic lupus erythematosus (SLE), Sjögren’s syndrome, systemic sclerosis, dermatomyositis, rheumatoid arthritis (RA), and ANCA-associated vasculitis (AAV). These diseases often involve multi-systemic involvement and the presence of various autoantibodies.
The pathogenesis is complex, involving genetic, environmental, infectious, and hormonal factors. Most autoimmune diseases are chronic, relapsing, and currently incurable. Thus, clinical treatment aims to relieve symptoms, reduce flares, and maintain patients’ quality of life.
🔹 Mainstream treatments include:
- Glucocorticoids: Powerful anti-inflammatory agents, often first-line during acute flares.
- Immunosuppressants (e.g., cyclophosphamide, methotrexate, leflunomide): Help control abnormal immune activity and reduce recurrence.
- Biologics (e.g., TNF-α inhibitors, IL-6 blockers, anti-CD20 antibodies): Used especially in refractory connective tissue diseases.
- Small-molecule JAK inhibitors: Offer oral, targeted regulation for select inflammatory conditions.
With advancements in research, autoimmune disease treatment is evolving from broad-spectrum immunosuppression to targeted modulation and personalized management, making long-term disease control and a return to normal life increasingly achievable.
CAR-T Therapy: From Targeted Clearance to Immune Rebuilding
In certain autoimmune diseases, pathogenic B cells are considered the culprits, producing autoantibodies and activating T cells via antigen presentation and cytokine release, driving systemic inflammation and organ damage.
CAR-T therapy involves isolating T cells from a patient, genetically modifying them to express chimeric antigen receptors (CARs) that recognize specific antigens (e.g., CD19 or BCMA), expanding them ex vivo, and reinfusing them into the patient. These engineered cells seek out and eliminate abnormal B cells, disrupting the disease process at its root.
Think of CAR-T as transforming regular soldiers into elite commandos equipped with radar—precisely eliminating the “enemy cells” that fuel inflammation and tissue injury.
Why CAR-T is superior to traditional B cell-targeted therapies:
- Anti-CD20 monoclonal antibodies like rituximab can suppress B cells short-term but fail to eliminate long-lived plasma cells or cells in tissues, leading to incomplete remission and relapse.
- CAR-T cells targeting CD19 or BCMA can deeply penetrate tissues and eliminate broader B cell subsets, offering a more durable response.
- More importantly, CAR-T therapy facilitates immune reconstruction. After pathogenic B cells are eliminated, the immune system begins producing healthy, naïve B cells—establishing a new, balanced immune homeostasis.
This "clearance + reconstruction" dual action makes CAR-T a potentially curative option in select B cell–mediated autoimmune diseases.
Clinical Advances in CAR-T for Autoimmune Diseases
Since 2011, China has been at the forefront of CAR-T research and early clinical translation. Today, China is a global leader in CAR-T manufacturing, mechanistic studies, and new indications.
Chinese teams have pioneered clinical trials in autoimmune diseases, achieving world-class results. Notably, a universal CAR-T study targeting polymyositis and systemic sclerosis was published in Cell and named one of Science’s Top 10 Breakthroughs of 2024—a major milestone that validated CAR-T’s efficacy in autoimmune diseases and broadened its therapeutic scope.
The landmark application of CAR-T in systemic lupus erythematosus (SLE) has opened doors to other B cell–driven autoimmune conditions, including:
- Systemic sclerosis
- Sjögren’s syndrome
- ANCA-associated vasculitis
- Myasthenia gravis
- Autoimmune hepatitis
- Membranous nephropathy
- Polymyositis
Diseases mediated primarily by T cells or non–antibody pathways—such as type 1 diabetes, ulcerative colitis, ankylosing spondylitis, and psoriasis—are currently less suitable for CAR-T therapy.
Most common CAR-T targets in autoimmune trials:
- CD19: Expressed throughout B cell development
- BCMA: Covers plasma cells and long-lived plasma cells
This enables CAR-T to deliver a full-spectrum immune reset, from progenitor to effector B cells.
Emerging Platforms: Beyond Autologous CAR-T
Beyond traditional autologous CAR-T, new formats are rapidly emerging:
|
Format |
Features |
|
Autologous CAR-T |
Patient-derived, high compatibility, stable in vivo expansion |
|
Universal (Allogeneic) CAR-T |
Donor-derived, off-the-shelf, ideal for patients unable to provide autologous cells |
|
CAR-NK |
Natural killer–based, with better safety and MHC-independence |
Based on clinical experience, autologous CAR-T currently offers the most stable efficacy in autoimmune diseases.
Patient Selection: Scientific Screening Is Key
While CAR-T shows immense promise, not all autoimmune patients are eligible. Careful mechanism-based selection and individualized assessment are essential for safety and efficacy.
Selection considerations:
- Disease must be primarily B cell– or plasma cell–driven
- Patient must be fit for lymphodepletion preconditioning
- Adequate T cell quality for autologous manufacturing; if not, universal CAR-T may be considered
- Effect onset is not immediate: The full "clearance-reconstruction-remission" cycle may take 3–6 months
- Close monitoring is required for autoantibody titers, complement normalization, and B cell reconstitution
- Steroids or immunosuppressants must be tapered gradually post-treatment to avoid rebound
Outlook: Toward a New Era in Autoimmune Therapy
With deeper scientific understanding and ongoing technological advances, CAR-T therapy is expanding its reach into refractory autoimmune diseases, offering a beacon of hope to patients with limited options.
As more high-quality clinical trials mature, and platforms become more scalable, precise, and accessible, CAR-T is poised to become a mainstream, personalized, and affordable option, ushering in a new era of autoimmune disease treatment.


Decoding the Blueprint of Life: CAR-T as a Precision Navigation System Starting from Hematologic Malignancies
In the vast landscape of cancer immunotherapy, CAR-T cell therapy represents a transformative innovation. According to Dr. Changsong Qi of Peking University Cancer Hospital and Beijing GoBroad Hospital, its remarkable efficacy in hematologic malignancies stems from highly specific tumor-associated antigens such as CD19 (in acute lymphoblastic leukemia) and BCMA (in multiple myeloma), which serve as precise "navigation systems" for T cells.
"Essentially, the CAR construct reprograms T cells, enabling them to identify and eliminate tumor cells," explained Professor Qi. Once reinfused, these engineered T cells are able to precisely target and destroy tumor cells expressing these markers, achieving high response rates and sustained immune surveillance — a principle now validated by multiple commercial products and national regulatory frameworks.
The Challenge of Solid Tumors: Redefining the Role of CAR-T Therapy
Compared with hematologic cancers, the treatment of solid tumors is significantly more challenging, primarily due to:
- The lack of ideal, broadly expressed tumor-specific targets
- A highly immunosuppressive tumor microenvironment
- Tumor heterogeneity and antigen escape
“We initially explored targets like EGFR, but early studies showed an objective response rate of less than 10%, coupled with severe toxicity,” Professor Qi acknowledged. However, breakthroughs have since emerged with novel targets in solid tumors — including Claudin18.2 (gastric cancer), GPC3 (hepatocellular carcinoma), and GUCY2C (colorectal cancer).
Claudin18.2 has proven to be one of the most promising targets in gastric cancer, with expression in nearly 60% of moderate-to-high Claudin18.2-positive cases. CT041, a CAR-T product developed against this target, has entered Phase II clinical trials. Among over 200 patients treated, the objective response rate has consistently ranged between 40–50% — more than tenfold higher than the 2–5% seen with third-line standard treatments.
In liver cancer, GPC3 is expressed in approximately 70% of hepatocellular carcinomas and is nearly absent in normal tissues, making it an ideal “window target” for CAR-T therapy. New generations of structurally optimized GPC3-CAR-T products have achieved objective response rates exceeding 70% in mid-to-high dose cohorts, significantly prolonging median survival.
For colorectal cancer, GUCY2C (also known as GCC) shows stable expression in 60–70% of patients across both primary and metastatic sites. Professor Qi’s team is collaborating with industry partners to advance CAR-T products targeting GUCY2C, with early studies showing objective response rates of approximately 40%.
Innovative Delivery Routes: Paving the Way for CAR-T Cells to Reach Solid Tumors
A key determinant of CAR-T efficacy lies in the ability to physically deliver cells to tumor sites. “Traditional intravenous infusion often proves inadequate for certain locations,” Professor Qi emphasized. “Targeted delivery strategies are essential.”
Several innovative administration routes are currently being explored in clinical settings:
- Intraventricular injection: For intracranial tumors like neuroblastoma, CAR-T cells are infused directly into the brain ventricles to bypass the blood-brain barrier, enhance local effects, and minimize systemic toxicity.
- Hepatic arterial infusion: Especially for liver cancers expressing GPC3, CAR-T cells are delivered through the hepatic artery to achieve high local concentrations.
- Peritoneal/pleural infusion: Applicable to peritoneal metastases or thoracic tumors to maximize local efficacy.
In combination with debulking surgery, these approaches reduce tumor burden and improve the tumor microenvironment, ultimately enhancing CAR-T cell persistence and function.
Clinical Cases: Witnessing the Power of a “Living Drug”
Dr. Qi shared several compelling clinical cases that vividly illustrate the life-altering potential of CAR-T therapy as a “living drug”:
- A gastric cancer patient with anastomotic recurrence who was unable to eat regained the ability to consume porridge within just over a week following Claudin18.2-CAR-T infusion. One month later, rapid tumor regression even triggered bleeding — a dramatic confirmation of CAR-T's potent cytotoxicity.
- Another patient, previously fully reliant on parenteral nutrition due to complete obstruction, resumed oral intake just seven days after treatment and went on to enjoy over two years of high-quality life.
- A woman in her 30s, suffering from peritoneal metastases, renal failure, and carrying four drainage tubes, had all tubes removed within six months post-treatment and returned to work.
These examples demonstrate that CAR-T therapy is evolving from an experimental intervention into a modality capable of reconstructing lives.
Common Questions Answered: Helping More Patients Take the First Step
At the end of the interview, Professor Qi addressed several of the most pressing questions raised by patients:
On eligibility for clinical trials:
“Not all patients can enter clinical studies immediately,” he emphasized. “Target antigen testing is essential. Only when expression is confirmed can tumor burden and metastatic status be evaluated for trial inclusion.”
On 'pseudo-progression' post-treatment:
Some patients may initially show tumor enlargement on imaging. This is often due to CAR-T cell infiltration and inflammation rather than true disease progression. Imaging density and clinical symptoms are key to distinguishing between the two.
On timing and treatment duration:
“Unlike chemotherapy, CAR-T therapy involves a single infusion,”Professor Qi explained. “If the patient is stable, we recommend allowing time for the cells to act, rather than rushing into further treatment decisions.”
Closing Words: From Breakthrough to Cure, the Future of CAR-T Therapy Is Worth Pursuing
Dr. Qi concluded with an encouraging message:“Today, we are no longer content with simply prolonging survival — we are striving for true functional cures. CAR-T therapy is opening up new possibilities in the treatment of solid tumors. As long as we keep exploring and hold on to hope, the dawn of better outcomes will surely come.”


Jing Pan, Yue Tan, Lingling Shan, Samuel Seery, Biping Deng, Zhuojun Ling, Jinlong Xu, Jiajia Duan, Zelin Wang, Kai Wang, Xinjian Yu, Qinlong Zheng, Xiuwen Xu, Guang Hu, Taochao Tan, Ying Yuan, Zhenglong Tian, Fangrong Yan, Yajing Han, Jiecheng Zhang & Xiaoming Feng
Abstract
Refractory or relapsed T cell acute lymphoblastic leukemia (r/r T-ALL) patients have poor prognoses, due to the lack of effective salvage therapies. Recently, CD7-targeting chimeric antigen receptor (CAR)-T therapies show efficacy in patients with r/r T-ALL, but relapse with CD7 loss is common. This study evaluates a CD5-gene-edited CAR-T cell therapy targeting CD5 in 19 r/r T-ALL patients, most of whom had previously failed CD7 CAR-T interventions. CAR-T products were derived from previous transplant donors (Cohort A) or newly matched donors (Cohort B). Primary endpoints were dose-limiting toxicity at 21 days and adverse events within 30 days. Secondary endpoints were responses, pharmacokinetics and severe adverse events after 30 days. A total of 16 received infusions, 10 at target dose of 1 × 106 kg−1. All encountered grade 3–4 cytopenias and one had a grade 3 infection within 30 days. All patients (100%) achieved complete remission or complete remission with incomplete blood count recovery by day 30. At a median follow-up of 14.3 months, four received transplantation; three were in remission and one died of infection. Of 12 untransplanted patients, 2 were in remission, 3 relapsed, 5 died of infection and 2 of thrombotic microangiopathy. CAR-T cells persisted and cleared CD5+ T cells. CD5− T cells, mostly CD5-gene-edited, increased but remained below normal levels. These results suggest this CD5-specific CAR-T intervention has a high remission rate for T-ALL patients. Evidence also suggests the risk of late-onset severe infection may be mitigated with consolidative transplantation. This study provides insights that could help to optimize this promising intervention.
Refer to the original: https://doi.org/10.1038/s41591-024-03282-2














